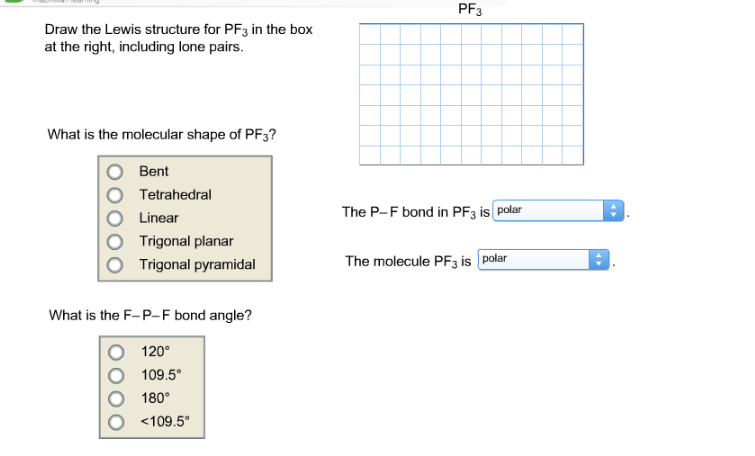
Draw the Lewis structure for PF3 in the box at the right including the lone pairs. Nine on three Br atoms and one on one phosphorous atom.
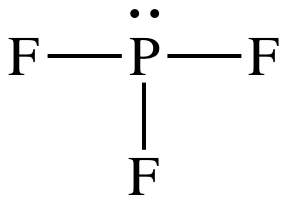
Chapter 11 Molecular Geometry Polarity Of Molecules And Advanced Bonding Theory
To sketch the PF3 Lewis structure by following these instructions.
. Draw the Lewis structure for PF3 in the box at the right including the lone pairs. PF3 Draw the Lewis structure for PF3 in the box at the right including lone pairs. March 10 2022 March 2 2022 by Admin.
The sp orbital with the lone pair on Plg is larger than on PF3. Thus the total lone electron pairs present on PBr3 lewis structure is ten ie. In io3- lone pair present on I atom 7-52 ie.
Draw the lewis structure for pf3. Draw a Lewis structure for each of the following compounds. Lewis Structure of PF3 for counting valence electrons around the terminal fluorine atoms.
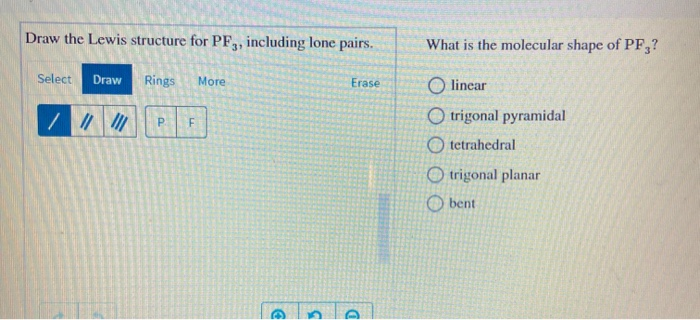
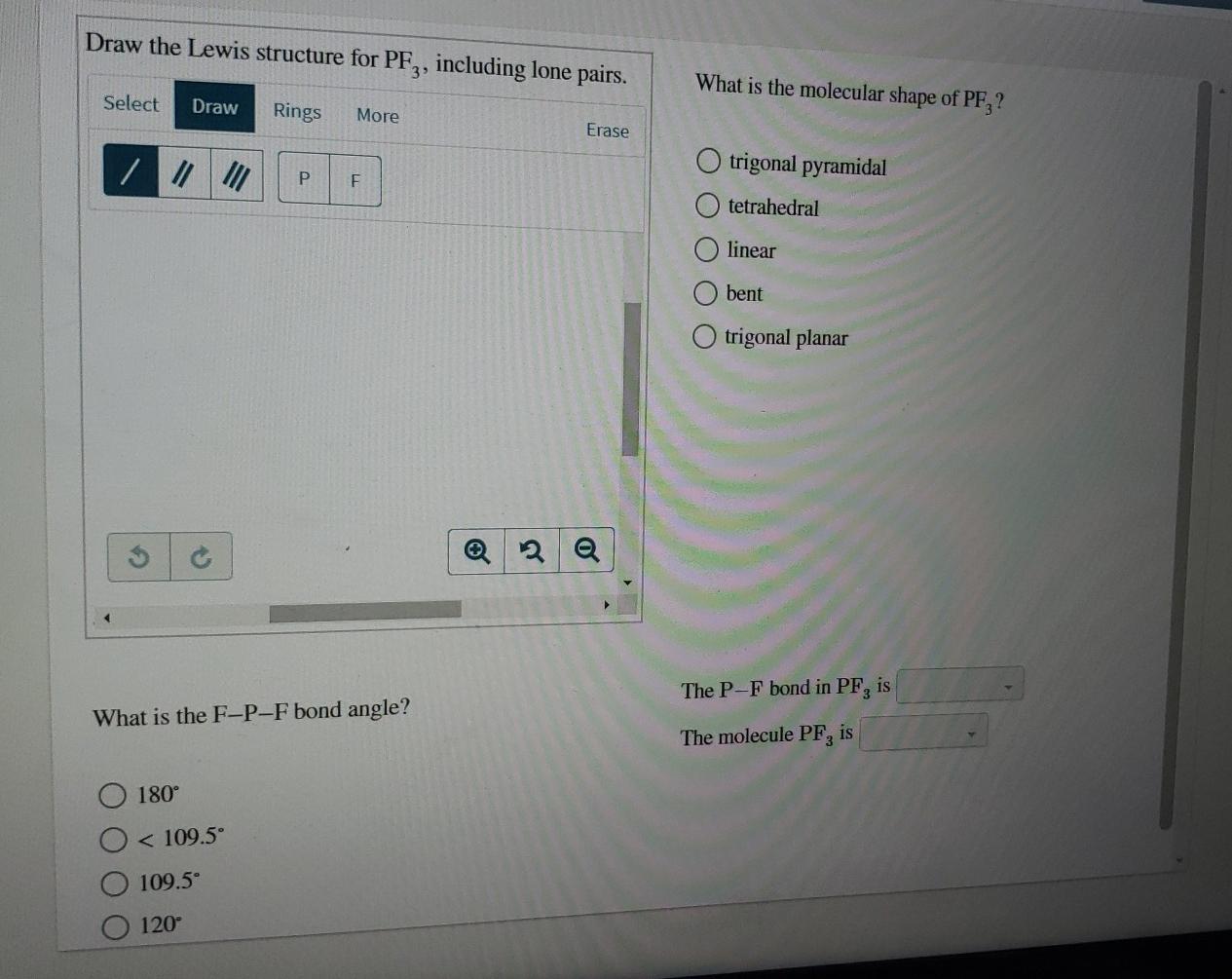
Select Draw Rings More Erase trigonal pyramidal bent F O trigonal planar linear O tetrahedral What is the F-P-F bond angle. 1 - Draw the Lewis structure of AsF4. Chemistry plays an essential role in the world of science showing the venue effect between the articles of the molems.
Lewis Structure of PH3 for constructing around the central Phosphorous atom. The P-F bond in PF3 is 1095 The molecule PF is 180 120 1095. S atom in SF4 is bonded to four Fluorine atoms and has one lone pair S has 6 valence electrons.
C 2 F 4. Lewis dot structure of PF3 contains 1 lone pair on. Both can act as Lewis bases.
Determine which statement best describes the Lewis acid or base properties of the compounds. Get the detailed answer. Linear Bent Trigonal planar Trigonal pyramidal Tetrahedral What is the F-B-F bond angle.
If you can do those Lewis structures PF 3 will be easy. 180 degree 120 degree 1095 degree. What is the molecular shape of BF_3.
PH3 Lewis dot Structure by counting valence electrons on the Phosphorous atom. 1 point for each structure. PF3 Draw the Lewis structure for PF3 in the box at the right including lone pairs.
The VSEPR shape of the molecule PF3 is trigonal pyrimidal. So all the three outer bromine atoms have total nine lone electron pairs in PBr3 lewis structure. Draw the Lewis structure for PF including lone pairs.
Lewis dot Structure for PH3 generated from step-1 and step-2. PF3 Lewis dot Structure by counting valence electrons on the phosphorus atom. To draw the Lewis structure of PF3 P F 3 we first count for the valence electrons of the compound.
The molecular geometry or shape for PF3 is the trigonal pyramid. In the lewis structure of PF 3 there are three single bonds around the phosphorus atom with three fluorine atoms attached to it. To sketch the PH3 Lewis structure by following these instructions.
The whole is the most crucial part of a chemical element breaking that we find plots tronses and non-Nutrons. 3 What is the molecular shape of PF. It is the most common and easiest way for scientists to draw electron configurations of atoms and ions.
Pf3 Lewis structure is a type of Lewis diagram that can be used to show how electrons are distributed in an octet. The diagrams can also be used to represent molecules such as water H2O or methane CH4. Similarly the central phosphorous atom in PBr3 lewis structure contains one lone pair of electron on it.
PF 3 is similar to PBr 3 and PCl 3. O PF3 and Plg both have phosphorus sp hybridized with a lone pair in an sp orbital. Include all lone pair electrons.
3 bonding pairs and one lone pair or. Three pairs will be used in the chemical bonds between the P and F. Up to 256 cash back Get the detailed answer.
Draw the Lewis structure for PF3 in the box at the right including lone pairs. 834 use lewis symbols and lewis structures diagram formation of pf3. What is the molecular shape of PF3.
Drawing the Lewis Structure for PF. Write a Lewis structure for the phosphorus trifluoride molecule PF3. Each fluorine atom has three lone pairs and the phosphorus atom has one lone pair.
Draw the Lewis structure of PF3 and Plg. LIMITED TIME OFFER. PF 3 phosphorus trifluoride has one phosphorus atom and three fluorine atoms.
Lewis dot Structure for PF3 generated from step-1 and step-2. In the PF 3 Lewis structure Phosphorus P is the least electronegative so it goes in the center. The Lewis structure of PF3 shows that the central phosphorus atom has how many non bonding and how many bonding electron pairs.
3 bonding pairs and one lone pair or non-bonding pair. See the answer See the answer done loading. Three pairs will be used in the chemical bonds between the P and F.
Lone pair present on each double bonded O atom6-24 ie. GET 20 OFF GRADE YEARLY SUBSCRIPTION. Lewis dot structure of PF3 contains 1 lone pair on the central atom phosphorous and 3 lone pairs on each outer atom fluorine.
The electron geometry for PF3 is tetrahedral as it central has 4 regions of electron density. These lone pair of electrons is found In the lewis structure of io3- on the given atoms as electron dots. Draw the Lewis structure for BF_3 in the box at the right including lone pairs.
Four of them went in bonding with four Fluorine atoms while the other two remained as a lone pair on S atom. 1 hour agoChemistry questions and answers. The Sulfur tetrafluoride is a polar molecule sf4 polar or nonpolar.
Lone pair present on single bonded O- ion 8-26 ie3 lone pair. In the Lewis structure for PF 3 there are a total of 26 valence electrons.

What Is The Molecular Geometry Of The Pf3 Molecule
Solved Draw The Lewis Structure For Pf Including Lone Chegg Com
Solved Pf3 Draw The Lewis Structure For Pf3 In The Box At Chegg Com
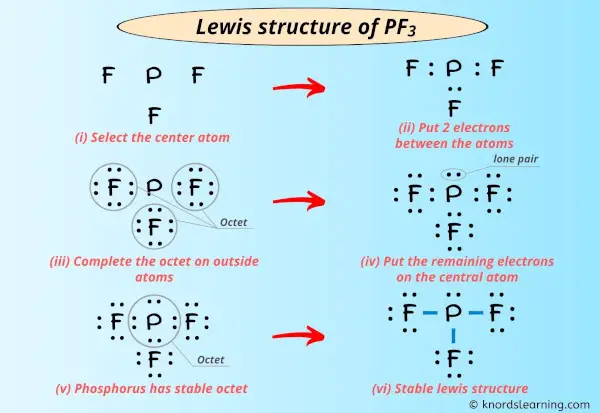
Lewis Structure Of Pf3 With 6 Simple Steps To Draw

Pf3 Lewis Structure How To Draw The Lewis Structure For Pf3 Youtube
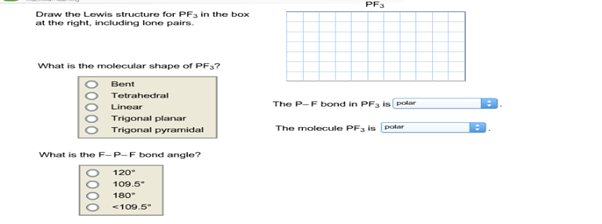
Get Answer Pf3 Draw The Lewis Structure For Pf3 In The Box At The Right Transtutors

Solved Draw The Lewis Dot Structure For Ch Cl Determine The Chegg Com

0 comments
Post a Comment